ODSMT Unveiled - The Science, Uses, And Potential Of Desmetramadol
Unlock the Potential of ODMST Medicine: Learn about Desmetramadol, the Opioid Analgesic That's Changing Pain Relief. Discover its benefits, uses, and how it differs from tramadol. Find your path to effective pain management with ODMST medicine today.
Author:Karan EmeryReviewer:James PierceNov 16, 20232.2K Shares63.6K Views
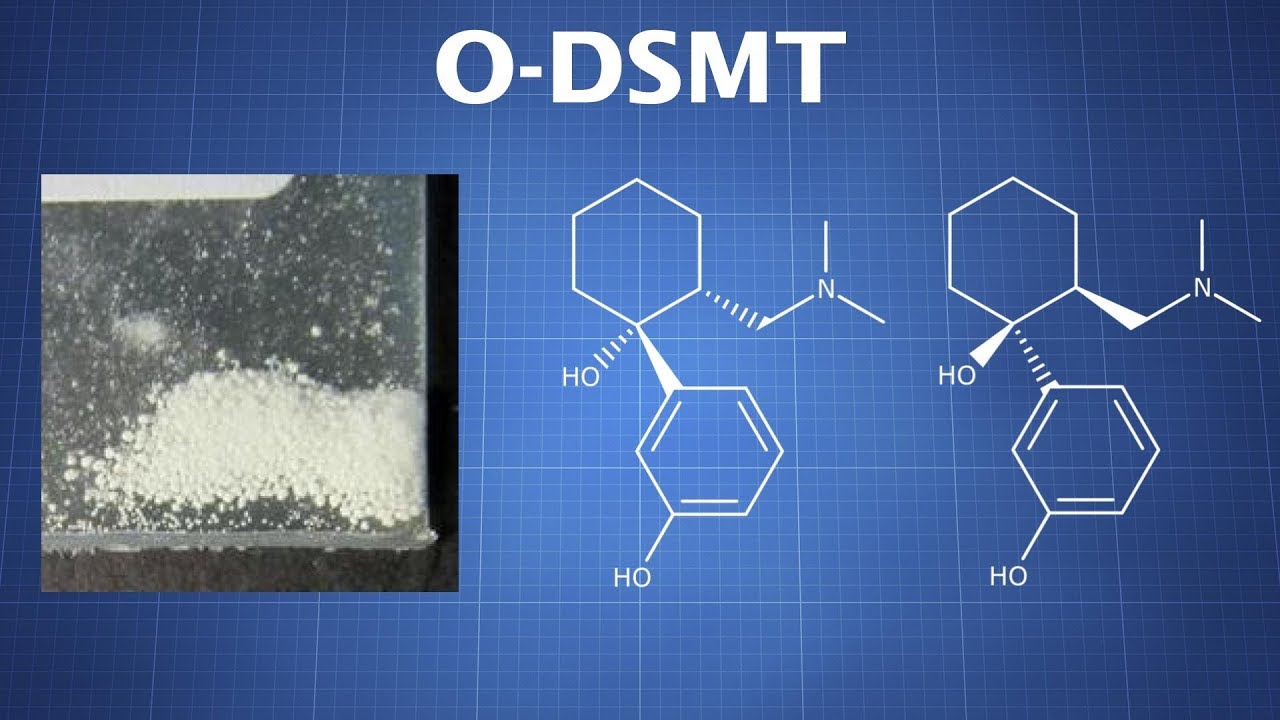
In the realm of pain management, ODMST, also known as O-desmethyltramadol, stands as a promising therapeutic agent. Derived from tramadol, ODMST possesses potent analgesic properties, effectively alleviating pain while minimizing the risk of addiction and dependence associated with traditional opioids. This remarkable compound has garnered significant attention from the medical community, paving the way for innovative pain relief strategies.
Unlike conventional opioids that primarily target mu-opioid receptors, ODMST exhibits a unique mechanism of action. It selectively binds to delta and kappa-opioid receptors, triggering a cascade of events that ultimately dampen pain signals. This selective binding profile contributes to ODMST's reduced addictive potential, making it a safer alternative for long-term pain management.
Uses And Potential Applications Of ODMST
While still under investigation, ODMST has demonstrated potential benefits over conventional opioids, including reduced risk of addiction and dependence, improved safety profile, and effective pain relief.
Current And Potential Uses Of ODMST
- Pain Management - ODMST is primarily being investigated for its potential as a safer and more effective alternative to conventional opioids in pain management. It has shown promise in treating various types of pain, including acute pain, chronic pain, and neuropathic pain.
- Reducing Opioid Dependence -ODMST's reduced addictive potential could make it a valuable tool for reducing reliance on conventional opioids and mitigating the risks of opioid dependence and addiction.
- Improving Safety in Pain Management -The improved safety profile of ODMST, particularly its lower propensity to induce respiratory depression, could offer a safer alternative for patients with respiratory conditions or those at risk of respiratory complications.
- Personalized Pain Management Strategies -ODMST could be integrated into personalized pain management approaches, tailored to individual patient characteristics and pain profiles. This could involve genetic testing to identify patients who may respond favorably to ODMST and optimize dosing based on individual metabolism and response.
- Combination Therapies -ODMST could be combined with other pain management modalities, such as non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or non-pharmacological interventions like physical therapy or cognitive-behavioral therapy. These combination therapies could provide synergistic pain relief and address different aspects of pain perception.
- Addressing the Opioid Crisis -The potential widespread adoption of ODMST could contribute to addressing the opioid crisis by providing a safer and less addictive alternative to conventional opioids. This could reduce the incidence of opioid-related overdoses, dependence, and misuse, leading to a significant public health benefit.
ODMST holds promising potential for various therapeutic applications, primarily in pain management. Its potential benefits, including reduced addictive potential, improved safety profile, and effective pain relief, could revolutionize pain management strategies and provide safer, more effective pain relief for patients worldwide.
Minimizing Adverse Effects And Respiratory Depression
Minimizing adverse effects and respiratory depression is crucial when using ODMSTor any other medication. Here are some strategies to minimize these risks:
- Tailored Dosing -Individualized dosing is essential to achieve optimal pain relief while minimizing side effects. Factors such as age, weight, medical history, and pain severity should be considered when determining the appropriate dosage.
- Close Monitoring -Regular monitoring by a healthcare professional is essential to assess the effectiveness of ODMST treatment and promptly address any adverse reactions. This includes monitoring for potential side effects, such as nausea, dizziness, and drowsiness.
- Patient Education - Comprehensive patient education is crucial to ensure proper use of ODMST and minimize the risk of misuse or abuse. Patients should be informed about the medication's potential benefits and risks, as well as proper dosage, administration, and storage guidelines.
- Adjunctive Therapies -ODMST can be effectively integrated with non-pharmacological pain management strategies, such as physical therapy, cognitive-behavioral therapy, and relaxation techniques. This holistic approach can enhance pain relief and promote overall well-being.
- Prevention of Misuse -Implementing safeguards to prevent misuse and diversion of ODMST is essential. This includes secure storage, prescription monitoring programs, and patient education on the dangers of misuse and addiction.
- Consideration of Alternatives -ODMST may not be suitable for all patients, and alternative pain management options should be considered based on individual needs and circumstances. These alternatives may include non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or other non-opioid analgesics.
- Continuous Evaluation -Ongoing evaluation of ODMST's effectiveness and safety is crucial. This includes monitoring patient outcomes, conducting clinical trials, and staying abreast of emerging research on the medication's benefits and risks.
How Is Tramadol Converted To O-desmethyltramadol?
Tramadol is converted to O-desmethyltramadol (ODSMT) through a process called demethylation, which involves the removal of a methyl group from the tramadol molecule. This metabolic transformation is primarily carried out by the cytochrome P450 (CYP) enzyme system, specifically the CYP2D6 enzyme.
The conversion of tramadol to ODMST occurs primarily in the liver, where CYP2D6 enzymes are abundant. Once tramadol is ingested, it enters the bloodstream and is transported to the liver. There, CYP2D6 enzymes catalyze the removal of a methyl group from the tramadol molecule, resulting in the formation of ODMST.
ODSMT is the main active metabolite of tramadol and is considered to be more potent than tramadol itself. It is responsible for a significant portion of tramadol's analgesic effects and is also thought to contribute to its side effects, such as nausea, dizziness, and drowsiness.
The rate of conversion of tramadol to ODMST can vary between individuals due to genetic polymorphisms in the CYP2D6 gene. Individuals with a reduced or absent CYP2D6 activity may experience less pain relief from tramadol and may be at a lower risk of side effects. Conversely, individuals with ultra-rapid CYP2D6 activity may experience more potent pain relief but may also be at a higher risk of side effects.
What Class Of Drug Is Tramadol?
Tramadol is classified as a centrally-acting analgesic, a type of pain medication that works by affecting the central nervous system (CNS). It is also considered an atypical opioid, as it has a unique mechanism of action that differs from traditional opioids like morphine or oxycodone.
Tramadol's analgesic effects are mediated by two primary mechanisms:
- Mu-opioid receptor agonist -Tramadol binds to mu-opioid receptors in the CNS, mimicking the effects of endogenous opioids like endorphins. This binding reduces the perception of pain signals and produces analgesic effects.
- Serotonin and norepinephrine reuptake inhibitor (SNRI) -Tramadol inhibits the reuptake of serotonin and norepinephrine, two neurotransmitters involved in pain modulation. This action increases the availability of these neurotransmitters in the CNS, contributing to pain relief.
Due to its dual mechanism of action, tramadol is considered a weak opioid agonist, meaning it has a lower affinity for mu-opioid receptors compared to traditional opioids. This lower affinity translates to a reduced risk of addiction and dependence, but it also means that tramadol may not be as effective for severe pain as stronger opioids.
Tramadol is typically prescribed for moderate to moderately severe pain, and it is often used as an alternative to traditional opioids when there are concerns about addiction or dependence. It is available in various formulations, including oral tablets, capsules, and extended-release formulations.
Despite its classification as an atypical opioid, tramadol still carries a risk of side effects, including nausea, dizziness, drowsiness, and constipation. It can also interact with other medications, particularly those that affect serotonin levels, such as antidepressants.
Will ODMST Make You Sleepy?
Yes, both tramadol and its active metabolite, O-desmethyltramadol (ODSMT), can cause drowsiness as a common side effect. This drowsiness is primarily due to their action on the central nervous system (CNS), particularly their interaction with certain neurotransmitters involved in sleep regulation.
Tramadol and ODMST can bind to mu-opioid receptors in the CNS, which are involved in pain modulation and also contribute to sedation. Additionally, they can inhibit the reuptake of serotonin and norepinephrine, two neurotransmitters that play a role in alertness and wakefulness. By increasing the levels of these neurotransmitters, tramadol and ODMST can indirectly induce drowsiness.
The degree of drowsiness experienced with tramadol and ODMST can vary between individuals due to factors such as individual sensitivity, dosage, and concomitant medications. Some people may experience mild drowsiness, while others may feel more significant sedation.
The drowsiness caused by tramadol and ODMST is generally more pronounced during the initial stages of treatment or when the dosage is increased. As the body adapts to the medication, the drowsiness may become less noticeable.
To minimize the risk of drowsiness and potential accidents, it is recommended to avoid driving or operating machinery while taking tramadol or ODMST. It is also advisable to avoid alcohol consumption, as it can further increase drowsiness. If drowsiness is interfering with daily activities, consult with your healthcare provider to adjust the dosage or consider alternative pain management strategies.
What Is The Difference Between Tramadol And Slow Release Tramadol?
The main difference between tramadol and slow-release tramadol lies in their absorption and release mechanisms, which affect their duration of action and dosing frequency.
- Tramadol -Regular tramadol is typically formulated as immediate-release tablets or capsules. Once ingested, the medication is rapidly absorbed into the bloodstream, reaching peak concentrations within 2-3 hours. This rapid absorption leads to a quick onset of pain relief, but the effects wear off relatively quickly, requiring more frequent dosing, usually every 4-6 hours.
- Slow-release Tramadol -Slow-release tramadol, also known as extended-release or sustained-release tramadol, is designed to release the medication gradually over an extended period, typically 12 or 24 hours. This controlled-release mechanism results in a slower onset of pain relief, but the effects last longer, providing more consistent pain control and reducing the need for frequent dosing.
Comparing The Two
- Onset of Action -Regular tramadol has a faster onset of action, providing pain relief within 30-60 minutes, while slow-release tramadol takes longer to reach peak concentrations and provide noticeable pain relief.
- Duration of Action -Regular tramadol's effects last for 4-6 hours, requiring more frequent dosing, while slow-release tramadol provides pain relief for 12-24 hours, reducing the dosing frequency.
- Dosing Frequency -Regular tramadol is typically taken every 4-6 hours, while slow-release tramadol is taken once or twice daily, depending on the formulation.
- Blood Levels -Regular tramadol produces fluctuating blood levels, with peaks and troughs, while slow-release tramadol maintains more consistent blood levels over time.
- Applications -Regular tramadol is suitable for acute pain or breakthrough pain that requires rapid relief, while slow-release tramadol is better suited for chronic pain management, providing consistent pain control throughout the day or night.
Ongoing Research And Clinical Trials
Ongoing research and clinical trials are crucial for evaluating the efficacy, safety, and potential therapeutic applications of ODMST. These studies provide valuable insights into the medication's pharmacological properties, optimal dosing strategies, and its impact on various pain conditions.
Current Status Of ODMST Research
ODSMT is currently in the preclinical and early clinical development stages. Preclinical studies involve laboratory and animal experiments to investigate its pharmacological properties, mechanism of action, and potential side effects. Early clinical trials, typically Phase I and Phase II, focus on assessing the safety, tolerability, and pharmacokinetics of ODMST in human subjects.
Ongoing Clinical Trials
Several clinical trials are currently underway to evaluate the efficacy and safety of ODMST for various pain conditions. These trials are designed to compare ODMST to placebo or other pain medications, such as tramadol or conventional opioids. The primary outcomes of these trials include pain relief, functional improvement, and safety parameters.
Future Directions
As research on ODMST progresses, future clinical trials will focus on larger patient populations, longer treatment durations, and specific pain conditions. These trials will provide more comprehensive data on ODMST's effectiveness, safety, and potential for long-term use in pain management.
Potential Applications
The outcomes of ongoing research and clinical trials will determine the potential applications of ODMST in clinical practice. If proven safe and effective, ODMST could be used to treat various types of pain, including acute pain, chronic pain, and neuropathic pain. It could also play a role in reducing opioid dependence and addressing the opioid crisis.
Regulatory Approval
The approval of ODMST for clinical use will depend on the results of clinical trials and regulatory review processes. If clinical trials demonstrate its efficacy and safety, ODMST could receive approval from regulatory agencies, such as the U.S. Food and Drug Administration (FDA), for specific pain management indications.
Frequently Asked Questions About ODMST
What Are The Potential Risks Of ODMST?
Like all medications, ODMST may have some potential side effects, including:
- Nausea
- Dizziness
- Drowsiness
How Is ODMST Taken?
ODSMT is typically taken orally as a tablet or capsule. The dosage will vary depending on the individual's needs and medical condition.
What Are The Potential Interactions With ODMST?
ODSMT may interact with other medications, including antidepressants, sedatives, and other opioids. It is important to tell your doctor about all the medications you are taking before starting ODMST.
What Is Tramadol Alternate Name?
It is available under the brand names Ultram, Ultram ER, Conzip, and also as generics. Tramadol is also available in combination with the pain reliever acetaminophen under the brand name Ultracet and as generics.
Conclusion
O-desmethyltramadol (ODSMT), an active metabolite of tramadol, emerges as a promising therapeutic agent in the realm of pain management. Its unique pharmacological properties and potential advantages over conventional opioids have garnered significant attention from the medical community.
ODSMT offers several potential benefits, including effective pain relief, reduced risk of addiction and dependence, and an improved safety profile. Its reduced affinity for mu-opioid receptors, the primary targets responsible for addiction, suggests a lower potential for misuse and dependence. Moreover, ODMST's reduced propensity to induce respiratory depression offers a safer alternative for patients susceptible to respiratory complications.
Jump to
Uses And Potential Applications Of ODMST
Minimizing Adverse Effects And Respiratory Depression
How Is Tramadol Converted To O-desmethyltramadol?
What Class Of Drug Is Tramadol?
Will ODMST Make You Sleepy?
What Is The Difference Between Tramadol And Slow Release Tramadol?
Ongoing Research And Clinical Trials
Frequently Asked Questions About ODMST
Conclusion

Karan Emery
Author

James Pierce
Reviewer
Latest Articles
Popular Articles