KI Formula - A Window Into The Chemistry And Applications Of Potassium Iodide
Go beyond the KI formula and gain a comprehensive understanding of potassium iodide's significance in various fields, from medicine to industry and beyond.
Author:Rhyley CarneyReviewer:Paula M. GrahamNov 22, 202336.7K Shares490.5K Views

Potassium iodide (KI) is an ionic compound with the chemical KI formula. It is a white, crystalline solid that is soluble in water. KI is composed of two ions: the potassium cation (K+) and the iodide anion (I-). These ions are held together by electrostatic forces, forming a regular crystalline structure. The KI formula reflects the stoichiometric ratio of these ions, indicating that one potassium ion is combined with one iodide ion.
Potassium iodide appears as white, crystalline solids that are soluble in water. It has a characteristically bitter, salty taste. The compound exhibits high hydroscopic nature, meaning it readily absorbs moisture from the surrounding environment. KI's melting point is 681°C, and its boiling point is 1330°C. Its density is 3.12 g/cm³, indicating a relatively heavy substance.
The history of potassium iodide dates back to the early 19th century. In 1811, Bernard Courtois, a French chemist, accidentally discovered iodine while investigating the properties of seaweed ash. He observed a violet vapor emanating from the ash and subsequently isolated the element iodine. Later, Courtois combined iodine with potassium hydroxide to produce potassium iodide.
The discovery of potassium iodide marked a significant advancement in medicine. In 1822, Jean-Baptiste Boussingault, a French chemist and physiologist, recognized the importance of iodine in preventing goiter, a condition characterized by an enlarged thyroid gland. Potassium iodide was subsequently employed to treat iodine deficiency and its associated health problems.
Decoding KI Formula And Science Of Potassium Iodide
Delving Into The Structure And Bonding Of KI
The structure of potassium iodide (KI) is characterized by a regular arrangement of potassium and iodide ions held together by electrostatic forces. The potassium ions, with their single positive charge (K+), occupy the octahedral sites in the crystal lattice, while the iodide ions, with their single negative charge (I-), occupy the cubic sites. This arrangement results in a stable, repeating pattern of ions throughout the crystal.
The bonding in KI is considered ionic, as it arises from the transfer of electrons from potassium to iodine. Potassium, with its relatively low ionization energy, readily loses its outer electron to form a positively charged ion (K+). Iodine, with its high electron affinity, readily accepts this electron to form a negatively charged ion (I-). The electrostatic attraction between these oppositely charged ions forms the ionic bond in KI.
Understanding The Chemical Reactions Of KI
Potassium iodide (KI) participates in various chemical reactions due to its ionic nature. It readily reacts with other ionic compounds, undergoing double displacement reactions. In these reactions, the cations and anions of the reactants are exchanged to form new products. For instance, when KI is mixed with silver nitrate (AgNO3), a double displacement reaction occurs, resulting in the formation of potassium nitrate (KNO3) and silver iodide (AgI).
KI also reacts with certain non-metallic elements, such as chlorine (Cl2), in redox reactions. In these reactions, electrons are transferred from one species to another, resulting in a change in oxidation states. For example, when KI is dissolved in an aqueous solution containing chlorine, a redox reaction occurs, producing potassium chloride (KCl) and iodine (I2).
Examining The KI Formula In Relation To Other Compounds
Potassium iodide (KI) belongs to the family of alkali metal halides, a group of compounds formed by the combination of alkali metals (Group 1) and halogens (Group 17). These compounds share similar ionic structures and properties, exhibiting high melting and boiling points due to the strong electrostatic forces between the ions.
KI is closely related to sodium iodide (NaI), another alkali metal halide. Both KI and NaI exhibit similar ionic structures and properties, but KI has slightly higher melting and boiling points due to the larger size of the potassium ion compared to the sodium ion. This difference in ion size also affects the solubility of the two compounds. KI is more soluble in water than NaI, as the larger potassium ions can accommodate more water molecules around them.
Potassium iodide (KI) is a versatile compound with a rich history and diverse applications. Understanding its structure, bonding, and chemical reactions provides insights into its unique properties and the underlying principles of ionic compounds. By examining its formula in relation to other compounds, we can appreciate the interconnectedness of chemistry and its role in shaping the world around us.
KI - A Versatile Compound With Diverse Applications
KI In Medicine - Combating Iodine Deficiency And Beyond
Potassium iodide (KI) plays a crucial role in medicine, primarily in addressing iodine deficiency disorders (IDDs), a group of conditions caused by a lack of iodine in the diet. IDDs can lead to a range of health problems, including goiter, hypothyroidism, and cretinism. KI supplementation is an effective and inexpensive strategy to prevent IDDs, particularly in regions with iodine-deficient soils or water sources.
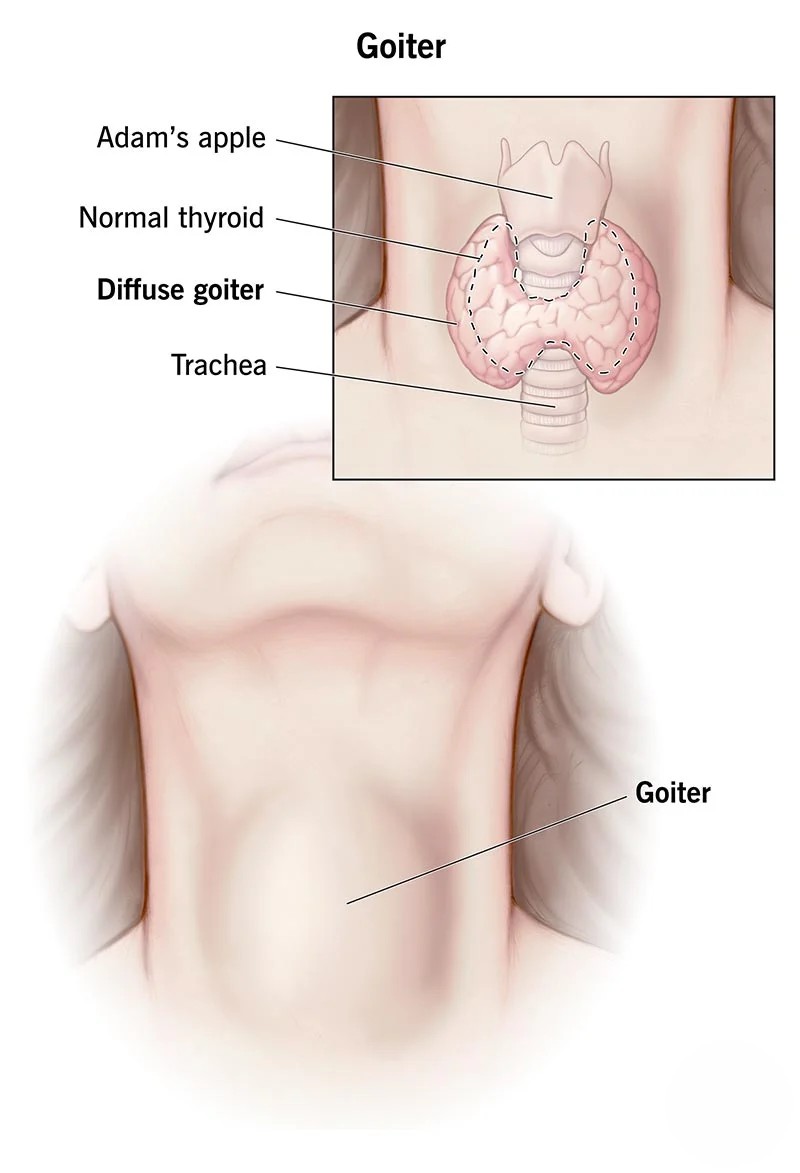
Apart from IDDs, KI has found applications in other medical areas:
- Thyroid protection -In the event of a nuclear accident involving radioactive iodine-131 (I-131), KI can be administered to saturate the thyroid gland with non-radioactive iodine, preventing the uptake of I-131 and minimizing the risk of thyroid cancer.
- Expectorant -KI can be used as an expectorant to help loosen mucus and make it easier to cough up. This property is particularly beneficial for respiratory conditions like bronchitis and pneumonia.
- Antimicrobial agent -KI exhibits weak antimicrobial properties, making it useful in certain topical applications, such as antiseptic solutions and disinfectants.
KI In Food Production - Enhancing Shelf Life And Preservation
Potassium iodide (KI) serves as a versatile food additive, enhancing shelf life and preservation in various food products:
- Salt fortification -KI is commonly added to table salt to combat iodine deficiency, particularly in regions with limited access to iodine-rich foods.
- Bread dough conditioner -In bread baking, KI acts as a dough conditioner, improving elasticity and preventing dough shrinkage.
- Fruit and vegetable preservation -KI can be used to preserve fruits and vegetables, particularly apples, bananas, and potatoes, by preventing browning and prolonging shelf life.
- Anti-caking agent -KI is used as an anti-caking agent in some food products, preventing clumping and ensuring a uniform texture.
KI In Industry - A Crucial Ingredient In Various Processes
Beyond its applications in medicine and food production, potassium iodide (KI) plays a vital role in various industrial processes:
- Photography -KI is used in photography to prepare silver halide emulsions, the light-sensitive coatings on photographic films and papers.
- Dyes and pigments -KI is employed in the production of various dyes and pigments, such as potassium iodide starch (KI starch), a sensitive starch-iodine complex used as a redox indicator.
- Metal refining -KI is utilized in the refining of certain metals, such as gold and silver, to remove impurities and improve purity.
- Synthesis of other chemicals -KI serves as a starting material in the synthesis of various chemicals, including potassium iodate (KIO3), a bleaching agent used in the textile industry.
Potassium iodide (KI) is a versatile and valuable compound with a wide range of applications in medicine, food production, and industry. Its unique properties and ability to participate in various chemical reactions make it an essential ingredient in numerous processes that impact our daily lives.
Managing KI - Storage, Safety, And Handling
Safe Handling And Storage Guidelines For KI
Potassium iodide (KI) is a generally safe compound, but it is essential to handle and store it properly to minimize potential risks. Here are some guidelines for safe handling and storage of KI:
Handling Precautions:
- Wear appropriate personal protective equipment (PPE) -When handling KI, it is advisable to wear gloves, goggles, and a lab coat to protect your skin and eyes from direct contact with the substance.
- Work in a well-ventilated area -KI is a hygroscopic compound, meaning it readily absorbs moisture from the air. Working in a well-ventilated area helps prevent the inhalation of KI dust or fumes.
- Avoid contact with incompatible substances -KI can react with certain substances, such as strong oxidizing agents, to produce hazardous compounds. Familiarize yourself with the hazards of incompatible substances and avoid mixing them with KI.
Storage Precautions:
- Store KI in airtight containers -Store KI in airtight containers to prevent moisture absorption and ensure the integrity of the compound.
- Keep KI away from heat and light -Store KI in a cool, dark place away from direct sunlight and heat sources. Exposure to heat and light can degrade KI and potentially produce hazardous byproducts.
- Label containers clearly -Clearly label containers containing KI with the substance's name, date of receipt, and any relevant hazard warnings. This information is crucial for proper handling and storage.
- Keep KI out of reach of children and pets -Store KI in a secure location out of reach of children and pets to prevent accidental ingestion or exposure.
Understanding Potential Hazards And Side Effects Of KI
While KI is generally safe when used as directed, it is essential to be aware of potential hazards and side effects:
- Skin irritation -Direct contact with KI can cause skin irritation, especially in individuals with sensitive skin. If skin contact occurs, wash the affected area thoroughly with soap and water.
- Eye irritation -Exposure to KI dust or fumes can cause eye irritation, including redness, watering, and discomfort. If eye contact occurs, immediately flush the eyes with plenty of clean water for at least 15 minutes.
- Digestive issues -Ingesting excessive amounts of KI can lead to digestive problems, such as nausea, vomiting, and diarrhea. If accidental ingestion occurs, seek medical attention promptly.
- Allergic reactions -In rare cases, individuals may experience allergic reactions to KI, characterized by symptoms such as hives, itching, and difficulty breathing. If you suspect an allergic reaction, seek medical attention immediately.
Adhering To Safety Regulations And Protocols
When working with KI, it is crucial to adhere to relevant safety regulations and protocols to protect yourself and others:
- Consult safety data sheets (SDS) -Before handling KI, thoroughly review the SDS for the specific product you are using. The SDS provides detailed information on the compound's hazards, handling precautions, and emergency procedures.
- Follow workplace safety guidelines -If working in a laboratory or industrial setting, follow the established safety guidelines and protocols for handling hazardous substances.
- Train personnel adequately -Ensure that personnel handling KI are adequately trained in safe handling procedures, emergency response measures, and the use of appropriate PPE.
- Dispose of KI properly -When disposing of KI or KI-containing waste, follow local and regulatory guidelines for the proper disposal of hazardous materials.
By adhering to these safety precautions and regulations, you can minimize the risks associated with handling and storing KI, ensuring a safe working environment for yourself and others.
People Also Ask
What Is The Difference Between Iodine And Iodide?
Iodine rarely occurs as an element but rather as a salt; for this reason, it is referred to as iodide and not iodine. Iodide is quickly and almost completely absorbed in the stomach and duodenum. Iodate is reduced in the gastrointestinal tract and absorbed as iodide [2,5].
What Is The Relationship Between Iodine And Iodide?
I-, iodide, is essentially the only form found in nature. Iodide is the ionic state of iodine, occurring when iodine forms a salt with another element, such as potassium. In this form, iodide can be ingested or applied topically (such as with povidone iodine, an iodide).
Is KI An Acid Or Base?
Potassium iodide (KI) will be neutral. Add universal indicator to a drop of this as well to see how it behaves in neutral solution. Now that you know the behavior of UI, use it to test the remaining solutions and record your observations. Keep track of whether each solution is acidic, basic, or neutral.
Conclusion
Potassium iodide (KI) is an ionic compound with the chemical KI formula. Potassium iodide (KI) is a versatile and valuable compound with a wide range of applications in medicine, food production, and industry. Its unique properties and ability to participate in various chemical reactions make it an essential ingredient in numerous processes that impact our daily lives.
From combating iodine deficiency and enhancing food preservation to playing a crucial role in photography and metal refining, KI's impact is far-reaching. Its versatility and diverse applications make it a testament to the power of chemistry and its ability to shape the world around us.

Rhyley Carney
Author

Paula M. Graham
Reviewer
Latest Articles
Popular Articles