Major Breakthrough for COVID-19 Vaccine
Where are we on the long-awaited COVID-19 vaccine? Is it ready to be released soon, and is it 100% safe and reliable? Pfizer has reached a significant milestone.
Author:Karan EmeryReviewer:Katharine TateNov 12, 202043.6K Shares1M Views
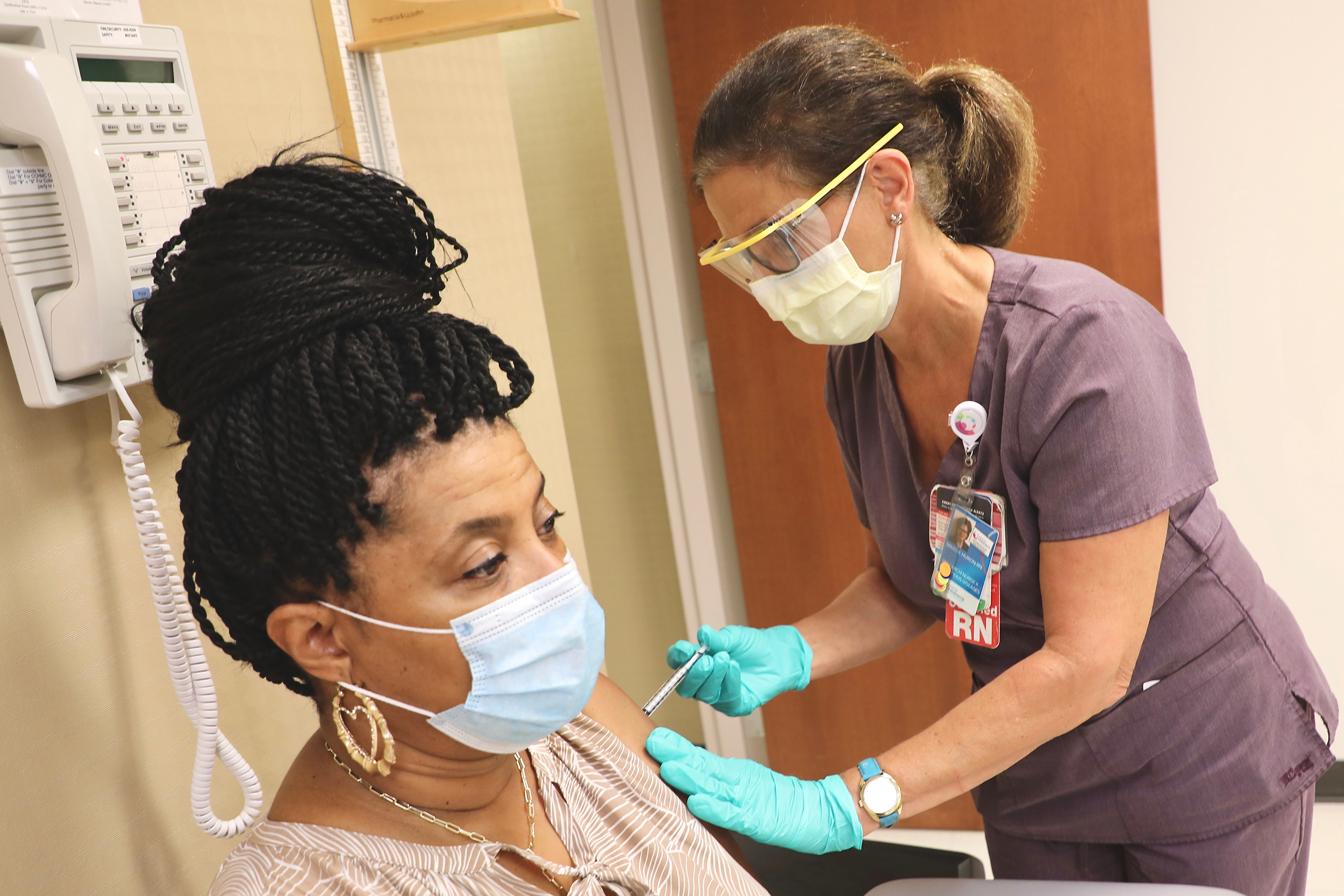
“Today is a great day for science and humanity. The first set of results from our phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent COVID-19,” said Dr. Albert Bourla, the Chairman and CEO of Pfizer, a well- respected company.
A Great Day For Science And Humanity
On November 09, 2020, Pfizerand BioNTechrevealed an important announcement. Their vaccine candidate against COVID-19 achieved the first interim analysis from phrase 3. The vaccine candidate called BNT162b2 is mRNA-based. BNT162b2 is discovered to have an efficacy rate of 90% against COVID-19. This is high among participants identified to have no prior evidence of SARS-CoV-2 infection.
The first interim efficacy analysis was conducted on November 08, 2020. This was done by an external, independent Data Monitoring Committee (DMC) from the phase 3 clinical study. It is a randomized, blind trial among the participants. They get a shot of the vaccine, and they don’t know whether they get the COVID-19 vaccine or not. The people who did were 90% less likely to get COVID-19 than those who got a placebo.
The Process
The companiesincluded in this breakthrough recently decided to drop the 32-case interim analysis. They began conducting the first interim analysis with a minimum of 62 cases.
The cases to be evaluated increased to 94 and the DMC performed its first analysis of all the cases. The case was divided into two. First, were the vaccinated participants and those who received the placebo. It indicates a vaccine efficacy rate above 90%, seven days after the second dose. This shows that protection from the virus can be possible to achieve after 28 days from the initiation of the vaccine.
There were over 43 538 participants enrolled in this successful study. The phase three clinical trials started on July 27, 42% of them are global participants, and 30% are from the U.S. Participants were chosen to have diverse racial and ethnic backgrounds.
Fortunately, there were no serious safety concerns that have been observed and identified. Although, safety and additional efficacy data are still in the process of the collection. This is as directed by the amount of safety data asked by the FDAin its guidance for potential Emergency Use Authorization. As of November 8, 2020, 38 955 of the participants received the second dose of the candidate vaccine.
Are The Vaccines Ready?
The study is not done yet and is still continuing on enrolling participants. It is expected to continue until the final analysis. Meaning, when a total of 164 confirmed COVID-19 cases have been accumulated over their estimated time frame. The participants in this study will be monitored for two years after the second dose of the vaccine. This is to ensure long-term protection and safety.
The candidate vaccine will also be further evaluated. Why? To see its potential of effectivity amongst those people who have prior exposure to SARS-CoV-2. Also, to provide prevention on severe cases of the virus as new mutationsare being reported in several countries.
New secondary endpoints evaluating the efficacy on cases accumulated for 14 days after the second dose will be added. But this should wait until the FDA gives their approval. This is along with the evaluation of the primary efficacy endpoints of the confirmed COVID- 19 cases accumulated from seven days after the second dose of the vaccine.
The addition of the second endpoints will give significant indications as the data across all the COVID-19 vaccine studies could be aligned and allow comparisons between these novel vaccines being created. Pfizer and Biotech are now working on the necessary safety and manufacturing data to be submitted to the FDA and demonstrate the safety and quality of the vaccine.
"
“We are reaching this critical milestone in our vaccine development program at a time when the world needs it most with infection rates setting new records, hospitals nearing- capacity, and economies struggling to reopen.
With today’s news, we are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis.” Dr. Bourla said during the announcement of the major success of the vaccine. They are now targeting to produce 50 million vaccine doses globally by the end of 2020 and up to 1.3 billion doses in 2021.
This major breakthrough proves something that hasn’t been confirmed for a while, which is that people can make a vaccine for COVID-19 that will provide strong immunityfrom the disease. The closer humanity to the vaccine, the more appropriate it does to not spread the virus.
It means that every person who will not fall sick to the virus in the next months to come is someone who might never get sick from this disease. The whole world has been suffering from this pandemic for not less than eight months already and is continually in misery because of the major setbacks caused by this global health crisis. Everyone has their hopes up for a vaccine that could end this grave situation that we have, and we can finally go back to our old normal.
However, there are still hesitationsbecause of the safety issues of the announced vaccines from major pharmaceutical companies. Safety and quality indeed should be the top priority because here lies the life of humanity. Pfizer and Biotech are working on this and holds a great promise on ending this global pandemic, saving millions of lives in the process.

Karan Emery
Author

Katharine Tate
Reviewer
Latest Articles
Popular Articles